Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Diabetic Kidney Disease: State of the Art
*Corresponding author: Christian David Pérez Calvo, Department of Medicine Intern, Free University, Colombia.
Received: June 17, 2022; Published: June 29, 2022
DOI: 10.34297/AJBSR.2022.16.002265
Summary
Diabetic kidney disease is one of the microvascular complications with the greatest impact on morbidity and mortality in patients with diabetes. Previously thought to be a linear series of events consisting of ultrafiltration, glomerular hypertension, albuminuria and successive decreases in GFR, it is now known to be affected by multiple metabolic and hemodynamic pathophysiological mechanisms, leading to cell signaling pathways, oxidative stress, deregulated autophagy, triggering structural damage and functional alterations that lead to disease. Risk factors for the disease that trigger pathophysiological mechanisms that contribute to its development are also recognized, such as obesity, smoking, poor metabolic control, arterial hypertension, ethnicity, among others. Although current therapies have not completely stopped the development of the disease, current efforts are focused on developing new therapies that can positively influence its onset and progression, with both SGLTi and AR-GLP1 showing a leading role, improving cardiovascular and cardiovascular outcomes. kidneys, regardless of their effect on the control of hyperglycemia, which is why they currently constitute a fundamental pillar of management. Finer none, a mineralocorticoid receptor antagonist, is another current therapy that has been shown to have an impact on cardiovascular and renal outcomes, playing a complementary role to ACE inhibitors and ARBs in the management of albuminuria.
Keywords: Diabetes Mellitus; Chronic Kidney Disease; Albuminuria; End-Stage Kidney Disease; Diabetic Nephropathy
Introduction
Diabetic Kidney Disease (DKD) is a microvascular complication that occurs in type 1 and type 2 diabetes, which increases morbidity and mortality compared to those who do not develop it, this condition often progresses to End-Stage Renal Disease (ESRD), in need of renal replacement therapy [1]. Traditionally considered as a series of sequential stages, beginning with hyperfiltration and glomerular hypertrophy, followed by albuminuria, which lead to impaired renal function. Currently, DN refers to the microangiopathic lesion produced by diabetes mellitus, mainly involving the glomerulus, characterized by persistent proteinuria, arterial hypertension and progressive worsening of the Glomerular Filtration Rate (GFR). It is recognized that a notable group of patients develop deterioration of the GFR without going through intermediate phases, for which the current paradigm that defines diabetic kidney disease is the deterioration of renal function. The term in current general use is diabetic kidney disease, since it is a broader definition that encompasses diabetic patients with renal compromise [2,3]. Strict glycemic control, Blood Pressure (BP) treatment, cardiovascular risk reduction and kidney protection measures focused on the Renin Angiotensin Aldosterone System (RAAS) can delay the progression and development of diabetic kidney disease (two).
Epidemiology
The international diabetes federation estimated a global prevalence of diabetes in adults aged 20-79 years in 2021 of 10.5%, for 2030 it projects an increase to 11.3% and for 2040 up to 12.2%. It is estimated that 240 million people have undiagnosed diabetes worldwide, this means that almost one in two adults with diabetes do not know they have it [4]. Microvascular complications that most impact the prognosis of patients with diabetes mellitus and the main cause of end-stage kidney disease. The incidence of DKD has doubled in the last decade due to the increase in cases of type 2 diabetes. It is currently estimated that about 25-35% of those diagnosed with type 2 diabetes already have microvascular complications [5]. Mortality from all causes in people with DKD is approximately 30 times higher than in diabetic patients who do not have it; most patients with DKD die of cardiovascular disease before developing end-stage renal disease [5].
Risk Factor’s
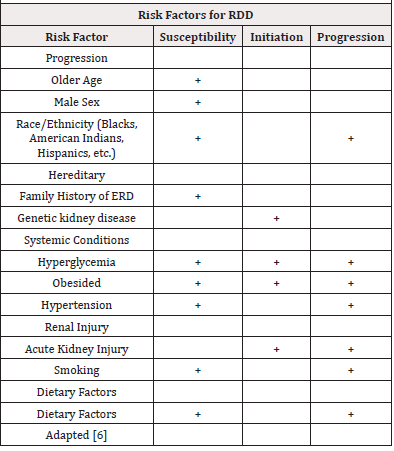
Risk factors for DKD are conceptually classified into susceptibility, initiation, and progression factors (Table 1), among which hyperglycemia and hypertension stand out [6,7]. In normoalbuminuric patients with type 1 diabetes, poor glycemic control has behaved as an independent predictor of progression to the development of albuminuria and/or ESRD [6,8]. Inadequate blood pressure control (SBP>140) in patients with type 2 diabetes has been widely associated with a higher risk of mortality and developing ESRD [9].
Pathophysiology
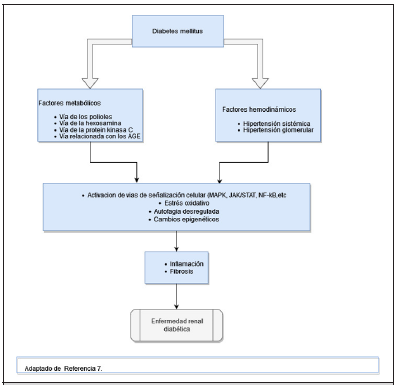
DRD has traditionally been explained as the result of the sum and relationship of hemodynamic and metabolic factors, however, it is now known that a complex network of events that determine kidney damage are involved in its development, including susceptibility factors (gender, race, age, family history and genetics), progression (diet, obesity, high blood pressure) and the initiating element that is hyperglycemia [10].
Hemodynamic Factors
Among the hemodynamic factors we have the increase in intraglomerular pressure and hyperfiltration, which occur in the early stages of diabetes and contribute to the development and progression of DKD. Ultrafiltration can be explained in part by unregulated tubuloglomerular feedback. In hyperglycemia, glucose hyperfiltration increases renal tubular reabsorption of glucose and sodium in the proximal tubule, resulting in reduced distribution of sodium to the distal tubular macula densa, leading to a marked decrease in resistance of the arterioles afferents and consequently to increased pressure in the glomerulus [11]. The increased pressure of the glomerulus produces mechanical stress on the capillary wall, which causes glomerulosclerosis and destruction of peritubular capillaries, it also increases the filtration of proteins into the tubular lumen, which causes the production of proinflammatory and profibrotic factors that lead to damage renal [11].
Metabolic Factors
Hyperglycemia activates metabolic pathways that generate Reactive Oxygen Species (ROS), such as the polyol pathway, the hexosamine pathway, the protein Pathway Kinase C (PKC) and the Advanced Glycation End products (AGE) pathway. ROS oxidize important macromolecules, including proteins, lipids and nucleic acids, ultimately leading to tissue damage [11,12]. In diabetes, mitochondrial function is altered, which also leads to an increase in ROS levels due to positive feedback of prooxidant enzymes such as Nicotinamide Adenine Dinucleotide Phosphate (NADPH). This accumulation of ROS and superoxide’s is considered the main trigger of events that lead to complications in diabetes mellitus, including DKD [13]. On the other hand, insulin resistance, high blood glucose levels and increased blood insulin levels independently cause endothelial dysfunction by promoting various intracellular mechanisms, such as increased production of Reactive Oxygen Species (ROS), Protein Kinase C (PKC) and advanced glycation product (AGE) inducing proinflammatory signaling [14]. Interactions of mediators produced by endothelial cells are disrupted and tend to become unbalanced. Among these mediators, Endothelin-1 (ET-1) is the most potent vasoactive peptide produced by endothelial cells to regulate vascular homeostasis. In endothelial cells, compensatory hyperinsulinemia increases ET-1 secretion, leading to vasoconstriction and vascular dysfunction. In the kidney, endothelin a receptor activation is associated in addition to vasoconstriction with podocyte damage, oxidative stress, inflammation and fibrosis [15,16].
Inflammatory Factors
Renal hypoxia and inflammation are also essential factors in the development of DKD. It is known that hypoxia is the result of an inadequate balance between oxygen requirements and supplies; In diabetes and hyperglycemia, the energy expenditure of the tubular cells is increased, which leads to an increase in the activity of the sodium-glucose cotransporter and glomerular hyperfiltration [17]. Although there is an increase in oxygen consumption, oxygen supply is decreased in DKD due to multifactorial causes, among which peritubular capillary loss and interstitial fibrosis stand out [18]. There are studies based on magnetic resonance imaging with oxygen level-dependent contrast imaging in animal models that have shown that the low level of oxygenation at the level of the renal cortex was an independent predictor of decreased renal function [19]. Inflammation plays a determining role in the developm of DKD due to various pathophysiological mechanisms; The independent relationship between C-Reactive Protein (CRP) and albuminuria in diabetic patients has been described. In this process, the role of multiple inflammatory cytokines as triggers of kidney damage in DKD stands out [20]. IL-1 is associated with an increase in the expression and assimilation of chemotactic factors and adhesins in the endothelial and mesangial cells, in addition to failures in the regulation of hyaluronic acid synthesis in the tubular epithelium and in the increase in endothelial permeability, as well as intraglomerular hemodynamic alterations [20,21].
For its part, IL-6 is associated with changes in endothelial permeability, mesangial cell proliferation and increased fibronectin expression. Renal IL-6 expression correlates directly with the severity and structural changes of renal hypertrophy and glomerular injury in DKD [20,21]. As for IL-18, this molecule is associated with increased albuminuria and changes in this parameter during kidney disease, suggesting that elevated levels of this cytokine may be a predictor of early kidney dysfunction in normoalbuminuric diabetic patients. [20,21]. TNFa also stands out, this molecule has biological activities that may be related to renal injury in diabetic patients: direct cytotoxicity to renal cells, induction of apoptosis, alteration of intrarenal hemodynamics, increased endothelial permeability or induction of oxidative stress. In experimental models of DM, renal levels of TNFa are elevated, which is associated with renal hypertrophy and hyperfiltration, initial alterations in the development of DRD [22,23]. Finally, the nuclear transcription factor kappa B (NF-Kb) stands out, which has been identified as a key player in the inflammatory pathways involved in DRD. This factor is in an inactivated form in the resident cells, however, before triggered stimuli. by hyperglycemia, AGEs, mechanical stress, proteinuria, angiotensin II, produce its activation, once activated it plays a central role in the activation and recruitment of cytokines, chemokines, and adhesion molecules [24,25].
Renin Angiotensin Aldosterone System
The activation and hyperactivity of the Renin Angiotensin Aldosterone System (RAAS) also play a key role in the deterioration of DKD, since there are multiple clinical trials and animal models showing that the inhibition of said system delays the progression of kidney damage in the ERD, it is also known that Angiotensin II and Transforming Growth Factor (TFG-B1) promote the development of renal fibrosis and renal tubular atrophy, induce chronic hypoxemia and promote microcirculation deterioration [26,27]. Aldosterone is also thought to play an important role in the pathophysiology of DRD by regulating gene expression and other mechanisms, including upregulation of prosclerotic growth factors such as Plasminogen Activator Inhibitor 1 (PAI-1) and TGF-B, and promotes macrophage infiltration and consequent renal fibrosis [27,28].
Disregulated Autophagy
Recent studies have shown that dysregulated autophagy also plays an important pathogenic role in DRD. Autophagy is the cellular degradation of macromolecules and organelles to preserve cellular homeostasis. Autophagy has two main physiological functions; one is to recycle intracellular resources according to nutrient requirements; another is the removal of damaged proteins and organelles under various stress conditions. [29,30]. The importance of autophagy in the pathogenesis of DRD was highlighted in a study using podocyte-specific autophagy-deficient mice, which showed decreased podocytes and massive proteinuria after a high-fat diet [31].
Genetics and Epigenetics
Recently, genetics and epigenetics have attracted great interest given that recent evidence shows that they play a relevant role in the pathophysiology of DRD. Among the epigenetic modifications, DNA methylation, non-coding RNAs, posttranslational modifications of histones stand out, which have been shown to be triggered by hyperglycemia, hypoxia, inflammation, and mediators such as cytokines [32]. These modifications determine that the cells of the individual acquire metabolic memory in such a way that although the patient obtains adequate control with medical interventions such as a proper diet, weight control and glycemia with drugs or insulin therapy, many patients continue to experience numerous complications associated with the ERD, suggesting the existence of a “memory” of prior exposure to hyperglycemia in target cells, leading to the persistence of its deleterious effects long after glycemic control has been established. Therefore, one of the current targets of approach and management is aimed at the development of epigenetic markers and modification therapies [33].
Diagnosis
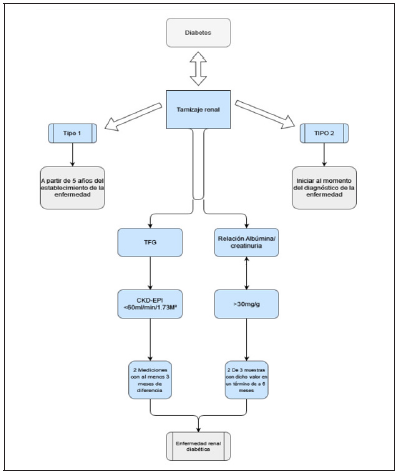
Diagnosis requires a history of confirmed diabetes and establishing the presence of kidney disease, which is based on two parameters: GFR and albuminuria [34,35]. Classically, the standard test to establish albuminuria as a marker of kidney damage has been the measurement of 24-hour urinary albumin excretion; however, the Albumin-Creatinuria ratio is currently used more frequently in a random urine sample. that the significant range is 30mg/g or higher, this value must be verified in at least 2 of 3 samples in a period of 3 to 6 months, given that physiological conditions such as exercise and inadequate hydration, or pathological conditions such as infections of the urinary tract and congestive heart failure could temporarily alter these values, leading to diagnostic inaccuracies [34,36,37]. Screening for albuminuria should begin at the time of diagnosis in type II diabetics and is recommended at 5 years in patients with type I diabetes mellitus, taking measurements annually [38] The GFR is the other parameter to establish kidney damage, which must be calculated with the validated formulas from the serum creatinine level, being the equation of the Chronic group Kidney Disease Epidemiology Collaboration (CKD-EPI) currently recommended, GFR levels persistently less than 60ml/min/1.73m2 are considered significant, for which two GFR measurements with a time interval of at least 3 months are required [38,39].
Treatment
Treatment aims to prevent complications associated with DKD, reducing disease progression and Cardiovascular Risk (CVR), which includes adequate control of glycemia, blood pressure, CVR and RAAS blockade. (two)
Non-Pharmacological Management of DRD
Recently, the KDIGO guidelines recommend a holistic approach that begins with the promotion of healthy lifestyles, these include a healthy diet, physical activity, tobacco cessation and weight control [40]. Tobacco use remains one of the leading causes of death worldwide and a known risk factor for developing CKD. Recent studies also underline the link between being a passive smoker and the development of the disease; The cessation of tobacco consumption should be recommended to all patients with DKD, avoiding passive exposure to tobacco smoke is another advisable intervention in these patients [40,41]. Regular exercise routines improve glycemic homeostasis, attenuate oxidative stress, optimize endothelial function, and modulate inflammation and RAAS. They have been considered a key strategy in achieving the goals of metabolic control and prevention of complications associated with diabetes mellitus, improving the quality of life of patients as well as having the potential to prevent and mitigate the development of DKD [42]. In general, regular exercise routines of moderate intensity are recommended, lasting at least 150 minutes/week, avoiding inactivity for 2 consecutive days, ideally, they should contain both aerobic and resistance exercises in adult patients with type 2 diabetes [43].
Genetics and Epigenetics
Dietary changes in DRD recommended by the Nutrition kidney foundation-KDOQI (NKF-KDOQI) comprise a calorie intake of 30- 35Kcal/kg/day, giving preference to foods such as whole grains, fibers, fruits, vegetables, and avoiding the intake of refined sugars. They recommend a sodium intake of less than 1.5 2.3g/day, the optimal amount of fat consumption is not yet completely clear in the ERD, however, it has been recommended to reduce the consumption of trans fats and saturated fatty acids, in the latter the guideline is <7% of the total daily calories, it has been shown that the consumption of polyunsaturated and monounsaturated fatty acids would have a protective effect in the attenuation of inflammation and endothelial dysfunction and would improve the control of blood pressure and lipids. Regarding the consumption of proteins in DRD, the recommendation has been to establish the intake according to the degree of renal compromise of the patients, in general it is recommended in patients with kidney disease Grade I-IV goals of 0.8/kg/day, for the on the contrary, in those who are on ESRD managed with renal replacement therapy, intakes >1.2g/ kg/day are considered appropriate [44]. Obesity is a risk factor for multiple health conditions including hypertension, cardiovascular disease, obstructive sleep apnea and osteoarthritis, classically the Body Mass Index (BMI) has been the measure used to measure this condition, however recent evidence suggests that the accumulation of fat in the waist or central obesity would also behave as a risk factor regardless of the BMI value. It is known that in obesity there are changes in renal structure and physiology, within the structural ones we find glomerulomegaly, mesangial expansion, sclerosis, and podocyte abnormalities, including a specific form of focal segmental glomerulosclerosis, within the functional ones hyperfiltration and proteinuria. Given that both DRD and obesity share characteristics in terms of anatomical and physiological alterations, it is considered that their association could have additive effects. Currently, all patients with obesity and DKD would be recommended to lose weight, especially those who are in non-advanced stages of the disease (I-III) [45,46].
Hyperglycemia
In patients with DKD, glycemic control plays a fundamental role in preventing the onset of the disease. The Action in Diabetes and Vascular Disease Trial: Preterax and Diamicron modified release-controlled Evaluation (ADVANCE) showed that intensive glucose control can reduce the development of microalbuminuria and macroalbuminuria [47]. The American Diabetes Association (ADA) suggests individualizing glycemic control goals according to the patient’s profile according to their age, comorbidities, and life expectancy, suggesting stricter control expectations (HbA- 1c<6.5%) in the young patient without comorbidities and with good life expectancy, as opposed to more lax goals (HbA-1c<8%) should be considered in the elderly patient with long-standing disease, macro and microvascular complications and shorter life expectancy [48-50]. The KDIGO management guidelines suggest an HbA-1c of less than 7% as a goal for diabetic patients with the aim of reducing and delaying the onset of microvascular complications, however, they clarify that in patients with a high risk of hypoglycemia, this goal should not be as strict, accepting values above 7% but less than 8% [39].
A Hypoglycemiating People
The current first-line pharmacological therapy in DRD is constituted in type 2 diabetic patients by metformin in association with an SGLT2i (sodium glucose cotransporter 2 inhibitors), provided that there is no contraindication for their administration, since they are drugs that impact both metabolic control and renal and cardiovascular outcomes, the addition and selection of other hypoglycemic agents when metabolic control goals are not achieved, must be established according to multiple factors such as the local availability of drugs, associated costs, patient preference, comorbidities, GFR levels, with GLP1-RAs being preferred by current evidence [40].
Metformin
Metformin is an oral hypoglycemic agent belonging to the biguanide group, considered a first-line agent in patients with type 2 diabetes, which has experimental studies that have shown to attenuate DRD by intervening in inflammatory mechanisms, oxidative stress, and fibrosis. In clinical trials, the use of metformin has been associated with a decrease in mortality, cardiovascular disease, and progression to ESRD, however, its use should be monitored and established with caution due to the increased risk of lactic acidosis [51]. In general, the use of metformin is not recommended in patients with advanced CKD, because an increase in mortality has been observed in these patients, its use is currently recommended in type 2 diabetic patients with GFR≥30ml/ min/1.73m², adjusting the dose according to renal function, being together with the SGLT-2i the first-line pharmacological therapy [51-53].
SGLT-2i
SGLT-2i prevent the reabsorption of filtered glucose at the renal tubular level, which triggers glycosuria by lowering blood glucose levels. They provide nephroprotective effects and reduce CVR in patients with DKD regardless of their hypoglycemic effect [54]. The mechanism by which they produce this effect is still not entirely clear. The main hypothesis focuses on urinary sodium excretion caused by the inhibition of sodium and glucose reabsorption in the proximal tubule. Increasing the sodium concentration in the macula densa, activating the tubuloglomerular feedback that would lead to vasoconstriction of the afferent arterioles and a reduction in intraglomerular pressure [55,56]. Regarding the adverse effects associated with SGLT-2i therapy, the most frequent are urogenital tract infections, with genital mycotic infection being the most frequent, especially in early phases of treatment, urinary tract infections are less common, with less still frequently, normoglycemic ketoacidosis may occur, educating the patient to avoid ketogenic diets, suspending use during acute illness, or prior to surgery could reduce the associated risks [57,58].
In the CREDENCE study, which compared canaglifozin vs. placebo in 4,401 albuminuric patients with type 2 diabetes and kidney disease, included patients with GFR>30ml/min/1.73m² and mean albumin/creatinuria ratio of 927mg/g. Canaglifozin showed a 34% decrease in the composite risk of doubling baseline serum creatinine, end-stage renal disease, or death from renal causes. (HR 0.66 (0.53 0.81); p<0.001). In addition, canaglifozin reduced three major adverse cardiovascular events (3p-MACE) by 20% (HR 0.80 (0.67-0.95), p<0.01) and hHF by 39% (HR 0, 61(0.47-90.8), p<0.001) [59]. The DAPA CKD study included patients with non-diabetic CKD, in patients with GFR 25-75ml/min/1.73m² and albuminuria, dapagliflozin demonstrated a 44% reduction in composite renal outcome, doubling baseline serum levels creatinine, ESRD, or mortality from renal causes (HR 0.56 (0.45-0.68); p<0.001) [60]. A meta analysis confirms the favorable effects of SGLT2i on the composite renal outcome of baseline serum creatinine doubling (40% decrease in GFR), initiation of Renal Replacement Therapy (RRT) or death from renal causes (RR 0.63(0.56-0.71) even in the presence of cardiovascular disease or multiple risk factors (RR 0.67(0.59-0.76) [61]. Based on these results, the use of canaglifozin, empaglifozin and dapagliflozin is currently supported as cardiovascular risk prevention and nephroprotective agents in the context of diabetic kidney disease, recommending their initiation in type II diabetic patients with GFR>20ml/min/1.73m² and have been suggested as first-line therapy in the KDIGO 2022 guidelines [40,62].
Glucagon-Like Peptide 1 Receptor Agonists
(AR-GLP-1)
Incretin hormone that stimulates insulin secretion in response to food intake, and its analogs are used to treat type 2 diabetes [63,64]. They are part of the agents with the potential to prevent DRD, however, not all those belonging to their group have shown such benefit, exendin-4 mimetic incretin analogs such as exenatide/ lixisenatide have not shown favorable results in cardiovascular outcomes [65] while the human AR-GLP1 such as liraglutide, dulaglutide and semaglutide have shown benefits [66,67]. In the LEADER and SUSTAIN-6 trials, treatment with liraglutide and semaglutide, respectively, was associated with a lower rate of major cardiovascular events or death [67,68]. In the REWIND trial of dulaglutide vs. placebo treatment in 9,901 participants where only 31% of participants had previously established cardiovascular disease, dulaglutide showed a reduction in 3p-MACE by approximately 12%, regardless of the pre-existing atherosclerotic cardiovascular disease. A meta-analysis of seven large clinical trials of GLP1-RA with 56,004 patients showed a 12% reduction in 3p-MACE. Composite renal outcomes were reduced by 17% for all GLP1-RAs, primarily due to a reduction in macroalbuminuria [66,69]. The cardiovascular and renal benefits provided by GLP1- RA drugs may be related to their direct effects on blood pressure, glucose, and body weight, as well as improving endothelial dysfunction and inflammation. Initially, they can cause a drop in GFR, which usually plateaus. Its use is currently recommended in patients with type 2 diabetes who do not achieve glycemic control goals or persist with albuminuria despite management with firstline therapy (Metformin+SLGTi) or as a substitute if the drug is not available or is contraindicated. use of these therapies. The use of long-acting drugs is recommended, starting with low doses, gradually increasing the dose to avoid gastrointestinal intolerance, combination with dipeptidyl inhibitors should be avoided peptidase 4 (iDPP-4) [40,70].
Arterial Hypertension
BP control in individuals with DKD has been shown to be essential in delaying the progression of the disease and reducing cardiovascular mortality. In the UK Prospective Diabetes Study (UKPDS), a decreased risk of microvascular complications was found up to 37% in patients who were treated with a blood pressure goal of <150/85mm Hg compared to those who were treated with a goal of <180/105mm Hg, in addition to the fact that for each increase of 10mmHg, an increase of 15% in the risk of developing Chronic Kidney Disease (CKD) Grade 3 evidenced either by the establishment of albuminuria or a doubling of serum creatinine levels [62,71]. Blood pressure goals have been controversial over time, the study The Systolic Blood Pressure Intervention Trial (SPRINT) in 2015 showed that, in patients with high cardiovascular risk, a decrease in systolic blood pressure (SBP) levels <120 vs <140 was associated with an improvement in cardiovascular and all-cause mortality, however these results could not be extrapolated to the diabetic population since this population was excluded from the stud [72]. The ACCORD- Blood studio pressure set out to investigate the effect of tight BP control on cardiovascular outcomes, particularly in diabetic patients at high risk for cardiovascular disease.
The results showed that lower BP goals (systolic blood pressure <120 vs <140) were associated with a lower risk of progression of proteinuria and stroke during the 5-year follow-up, but with no benefit for fatal cardiovascular events and non-fatal combined. Serious adverse events due to antihypertensive therapy were more frequent in the hypotensive group (3.3% vs. 1.3%) [73]. Initial treatment of hypertension in patients with diabetes should include lifestyle changes, including dietary sodium restriction (less than 2,300mg per day), weight loss if overweight or obese, increased activity fitness, moderating alcohol consumption and smoking cessation [74]. Currently the JNC 8 (Eighth Joint National Committee) and the KDOQI guidelines recommend BP goals <140/90 in diabetic patients regardless of the presence of CKD, if there is no proteinuria. In the presence of proteinuria, this parameter becomes stricter, with the goal <130/80 [75]. The agents of choice for blood pressure control in patients with DKD are Angiotensin Converting Enzyme Inhibitors (ACEI) and ANGIOTENSIN II RECEPTOR ANTAGONISTS (ARA II) [76,77], thiazide diuretics and Calcium antagonists appear as second-line agents in case BP goals are not achieved with ACE inhibitors or ARBs, since they seem to show cardioprotective effects, however they do not seem to have the same degree of benefit on the progression of DKD [78].
Blockade of the Renin Angiotensin Aldosterone System
RAAS blockade is an essential strategy for managing DKD. The benefit of RAAS blockade in DKD is independent of its effect on BP and is probably due to a greater extent to a reduction in intraglomerular pressure, impaired ultrafiltration, and resulting albuminuria. Because reduction in albuminuria is associated with slower DKD progression and better cardiovascular outcomes, control of albuminuria is an important goal of DKD treatment. In addition, RAAS inhibitors ameliorate angiotensin-induced oxidative stress, inflammation, and fibrosis. [72,79]. ACE inhibitors and ARBs are the agents with the most evidence and recommendation for use. Multiple randomized clinical trials with ACE inhibitors and ARBs have shown benefit in reducing the risk of doubling of serum creatinine and the composite outcomes of mortality and ESRD [80- 82]. The Diabetes Control and Complications Trial Research Study Group (DCCT) showed that captopril, independent of its effect on BP, reduced the risk of doubling serum creatinine levels by 48% in 3 years in individuals with type 1 DM and high levels of albuminuria [80].
The RENAAL (losartan) and IDNT (Irbesartan) studies showed that regardless of their effect on BP, the use of these ARBs showed a significant reduction in the composite outcome of doubling baseline serum creatinine, mortality, or renal failure in 16% and 20% respectively for approximately 3 years. [81,83]. The combined use of ACEI+ARA II has not been recommended since studies such as ONTARGET (Telmisartan + Ramipril) have shown that, although they produce lower levels of BP and albuminuria, they do not produce additional long-term benefit in relation to the monotherapy and in contrast significantly increased the risk of associated adverse events such as hyperkalemia and hypotension [84]. Mineralocorticoid Receptor Antagonists (MRAs) decrease albuminuria, however, there is a lack of evidence regarding the prevention of the development of ESRD, its most common adverse effects such as hyperkalemia have been prevented in long-term studies on DRD, strategies for clinical trials include the use of potassium chelators, aldosterone synthase inhibitors, and nonsteroidal mineralocorticoid receptor antagonists [85].
Finerenone
Among the promising therapies we find a non-steroidal MRA called Finerenone, the only one of its kind that has shown cardiovascular and renal benefits in two pivotal studies, especially caution should be exercised given the risk of developing hyperkalemia with this drug, however in case If it occurs, it could be managed with 72-hour breaks from the medication given its short half-life of action and if it does not improve with said measure, establish other strategies [86]. In the FIDELIO-DKD study (Finerenone in reducing kidney failure and disease diabetes progression kidney disease) has shown promising results, Finerenone achieved the primary endpoint (renal failure, a sustained decrease of at least 40% in GFR from baseline, or death from renal causes) in 17.8% of patients compared to 21.1% on placebo (HR 0.82 CI 073 to 0.93, p=0.001) [87,88]. In the FIGARO-DKD study, finerenone therapy improved cardiovascular outcomes compared to placebo in patients with type 2 diabetes who had CKD Grade 2-4 with moderately elevated albuminuria or CKD Grade 1 and 2 with severely elevated albuminuria. Therefore, finerenone may represent an important therapeutic option in DKD, currently recommending its use in the context of persistent albuminuria despite the use of an ACEI or ARA II, in patients with normal potassium [40,89].
Conclusion
microvascular complication of diabetes mellitus that leads to increased cardiovascular risk with a great impact on the morbidity and mortality of diabetic patients, its prevalence is on the rise as a result of the progressive increase in cases of diabetes mellitus, mainly type 2, it is currently recognized the ERD as a disease with dynamic and diverse pathophysiological mechanisms that occur simultaneously and not always linearly, where the markers that define the presence of the disease are GFR and albuminuria, however, in the future it is hoped that with a better understanding of pathophysiology even earlier markers develop. Current treatments can slow down the course of the disease, but not stop it completely, establish individualized guidelines according to the patient’s profile and their comorbidities, starting with changes in lifestyle, establish recommended first-line therapies up to interventions guided to specific objectives, impacting CVR, RAAS, glycemic control and BP, has been the most recommended approach.
Therapy with an ACE inhibitor or ARB continues to be the gold standard for blocking the RAAS axis. The reduction of cardiovascular risk is one of the most important goals in these patients, the most relevant innovative therapies are SGLT2i, and AR-GLP1, two groups of promising drugs, being agents with a very good safety profile, which in addition to contributing to glycemic control goals have been shown to have a favorable impact in available studies on both cardiovascular and renal outcomes. Finerenone, a non steroidal MRA, is another therapeutic option that has shown evidence in both renal and cardiovascular outcomes. It is necessary to continue conducting studies to better understand the pathophysiology, which will allow us to establish novel therapeutics that provide cardiovascular and renal benefits to improve the prognosis, prevention, and management of DRD in the future.
References
- Thomas B (2019) The Global Burden of Diabetic Kidney Disease: Time Trends and Gender Gaps. Curr Diab Rep 19(4):18.
- Umanath K, Lewis JB (2018) Update on Diabetic Nephropathy: Core Curriculum. American Journal of Kidney Diseases 71(6): 884-895.
- Vergara Arana A, Martinez Castelao A, Gorriz Teruel JL, Alvaro Moreno F, Navarro Gonzalez J, et al. (2020) Up-to-date nephrology. Diabetic Kidney Disease: Albuminuria and Progression.
- (2022) Home, Resources, diabetes L with, Acknowledgement, FAQs, Contact, et al. IDF Diabetes Atlas. 10th (Edn).
- Sagoo MK, Gnudi L (2020) Diabetic Nephropathy: An Overview. In: Gnudi L, Long D (Eds) Diabetic Nephropathy. Methods in Molecular Biology 2067: 3-7.
- Alicic RZ, Rooney MT, Tuttle KR (2017) Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin J Am Soc Nephrol 12(12): 2032-2045.
- Kazancioglu R (2013) Risk factors for chronic kidney disease: an update. Kidney International Supplements 3(4): 368-371.
- Caramori ML, Parks A, Mauer M (2013) Renal lesions predict progression of diabetic nephropathy in type 1 diabetes. J Am Soc Nephrol 24(7):1175-1181.
- Pohl MA, Blumenthal S, Cordonnier DJ, De Alvaro F, Deferrari G, et al. (2005) Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: clinical implications and limitations. J Am Soc Nephrol 16(10): 3027-3037.
- Navarro Gonzalez J, Mora Fernández C, Martinez Castelao A, Gorriz Teruel JL, Soler Romeo MJ, et al. (2020) Up-to-date nephrology. Diabetic kidney disease: etiopathogenesis and pathophysiology.
- Sugahara M, Pak WLW, Tanaka T, Tang SCW, Nangaku (M 2021) Update on diagnosis, pathophysiology and management of diabetic kidney disease. Nephrology (Carlton) 26(6): 491-500.
- Matoba K, Takeda Y, Nagai Y, Yokota T, Utsunomiya K, et al. (2020) Targeting Redox Imbalance as an Approach for Diabetic Kidney Disease. Biomedicines 8(2): 40.
- Cheng YS, Chao J, Chen C, Lv LL, Han YC, et al. (2019) The PKCβ-p66shc-NADPH oxidase pathway plays a crucial role in diabetic nephropathy. J Pharm Pharmacol 71(3): 338-347.
- Pérez-Morales RE, del Pino MD, Valdivielso JM, Ortiz A, Mora-Fernández C, et al. (2019) Inflammation in Diabetic Kidney Disease. NEF 143(1):12-16.
- Kohan DE, Barton M (2014) Endothelin and Endothelin Antagonists in Chronic Kidney Disease. Kidney Int 86(5): 896-904.
- Schneider JG, Tilly N, Hierl T, Sommer U, Hamann A, et al. (2002) Elevated plasma endothelin-1 levels in diabetes mellitus. Am J Hypertens 15(11): 967-972.
- Palm F, Cederberg J, Hansell P, Liss P, Carlsson PO (2003) Reactive oxygen species cause diabetes-induced decrease in renal oxygen tension 46(8): 1153-1160.
- Nangaku M (2006) Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol 17(1): 17-25.
- Rosenberger C, Khamaisi M, Abassi Z, Shilo V, Weksler-Zangen S, et al. (2008) Adaptation to hypoxia in the diabetic rat kidney. Kidney Int 73(1): 34-42.
- Navarro-González JF, Mora-Fernández C (2008) The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol 19(3): 433-42.
- Yaribeygi H, Atkin SL, Sahebkar A (2019) Interleukin-18 and diabetic nephropathy: A review. J Cell Physiol 234(5): 5674-5682.
- Navarro JF, Mora-Fernández C (2006) The role of TNF-alpha in diabetic nephropathy: pathogenic and therapeutic implications. Cytokine Growth Factor Rev 17(6): 441-450.
- Chen YL, Qiao YC, Xu Y, Ling W, Pan YH, et al. (2017) Serum TNF-α concentrations in type 2 diabetes mellitus patients and diabetic nephropathy patients: A systematic review and meta-analysis 186: 52-58.
- Tang SCW, Yiu WH (2016) Innate immunity in diabetic kidney disease. Nat Rev Nephrol 16(4): 206-222.
- Alicic RZ, Johnson EJ, Tuttle KR (2018) Inflammatory Mechanisms as New Biomarkers and Therapeutic Targets for Diabetic Kidney Disease. Adv Chronic Kidney Dis 25(2): 181-191.
- Gurley SB, Coffman TM (2007) The renin-angiotensin system and diabetic nephropathy. Semin Nephrol 27(2): 144-152.
- Lin YC, Chang YH, Yang SY, Wu KD, Chu TS (2018) Update of pathophysiology and management of diabetic kidney disease. J Formos Med Assoc 117(8): 662-675.
- Ritz E, Tomaschitz A (2009) Aldosterone a vasculotoxic agent-novel functions for an old hormone. Nephrol Dial Transplant 24(8): 2302-2305.
- Kume S, Koya D (2015) Autophagy: A Novel Therapeutic Target for Diabetic Nephropathy. Diabetes Metab J 39(6): 451-60.
- Tagawa A, Yasuda M, Kume S, Yamahara K, Nakazawa J, et al. (2016) Impaired Podocyte Autophagy Exacerbates Proteinuria in Diabetic Nephropathy. Diabetes 65(3): 755-767.
- Kim WY, Nam SA, Song HC, Ko JS, Park SH, et al. (2012) The role of autophagy in unilateral ureteral obstruction rat model. Nephrology (Carlton) 17(2): 148-159.
- Kato M, Natarajan R (2014) Diabetic nephropathy emerging epigenetic mechanisms. Nat Rev Nephrol. 10(9): 517-30.
- Kato M, Natarajan R (2019) Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nat Rev Nephrol 15(6): 327-345.
- Bonner R, Albajrami O, Hudspeth J, Upadhyay A (2020) Diabetic Kidney Disease. Prim Care 47(4): 645-659.
- J Rico et al (2021) Clinical practice guideline for diabetic kidney disease. Colombian Journal of Nephrology p. 5-27.
- Andrassy KM (2013) Comments on KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease». Kidney Int 84(3): 622-623.
- (2019) American Diabetes Association. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2019. Diabetes Care 42(Supp l 1): 124-138.
- (2020) American Diabetes Association. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2020. Diabetes Care 43(Suppl 1): 135-51.
- (2012) National Kidney Foundation. KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis 60(5): 850-886.
- KDIGO (2022) Clinical Practice guideline for diabetes management in chronic kidney disease. Public review draft 98(4): 1-115.
- Xia J, Wang L, Ma Z, Zhong L, Wang Y, et al. (2017) Cigarette smoking and chronic kidney disease in the general population: a systematic review and meta-analysis of prospective cohort studies. Nephrol Dial Transplant 32(3): 475-87.
- Amaral LS de B, Souza CS, Lima HN, Soares T de J (2020) Influence of exercise training on diabetic kidney disease: A brief physiological approach. Exp Biol Med 245(13): 1142-1154.
- Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, et al. (2016) Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 39(11): 2065-2079.
- Ko GJ, Kalantar-Zadeh K, Goldstein-Fuchs J, Rhee CM (2017) Dietary Approaches in the Management of Diabetic Patients with Kidney Disease. Nutrients 9(8): 824.
- Onyenwenyi C, Ricardo AC (2015) Impact of Lifestyle Modification on Diabetic Kidney Disease. Curr Diab Rep 15(9): 60
- Docherty NG, Canney AL, le Roux CW (2015) Weight loss interventions and progression of diabetic kidney disease. Curr Diab Rep 15(8): 55.
- Perkovic V, Heerspink HL, Chalmers J, Woodward M, Jun M, et al. (2013) Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney 83(3): 517-523.
- (2018) American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2018. Diabetes Care 41(Suppl 1): 55-64.
- Qaseem A, Wilt TJ, Kansagara D, Horwitch C, Barry MJ, et al. Hemoglobin A1c Targets for Glycemic Control With Pharmacologic Therapy for Nonpregnant Adults With Type 2 Diabetes Mellitus: A Guidance Statement Update From the American College of Physicians. Ann Intern Med 168(8): 569-576.
- McGrath K, Edi R (2019) Diabetic Kidney Disease: Diagnosis, Treatment and Prevention. Am Fam Physician 99(12): 751-759.
- Kawanami D, Takashi Y, Tanabe M (2020) Significance of Metformin Use in Diabetic Kidney Disease. Int J Mol Sci 21(12): 4239.
- Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK (2014) Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA 312(24): 2668-2675.
- Hung SC, Chang YK, Liu JS, Kuo KL, Chen YH, et al. (2015) Metformin use and mortality in patients with advanced chronic kidney disease: national, retrospective, observational, cohort study. Lancet Diabetes Endocrinol 3(8): 605-614.
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, et al. (2015) Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 373(22): 2117-2128.
- Fioretto P, Zambon A, Rossato M, Busetto L, Vettor R (2016) SGLT2 Inhibitors and the Diabetic Kidney. Diabetes Care 39 Suppl 2: 165-171.
- DeFronzo RA, Reeves WB, Awad AS (2021) Pathophysiology of diabetic kidney disease: impact of SGLT2 inhibitors. Nat Rev Nephrol. mayo de 17(5): 319-334.
- Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, et al. (2015) Euglycemic Diabetic Ketoacidosis: A Potential Complication of Treatment With Sodium-Glucose Cotransporter 2 Inhibition. Diabetes Care 38(9): 1687-1693.
- Thong KY, Yadagiri M, Barnes DJ, Morris DS, Chowdhury TA, et al. (2018) Clinical risk factors predicting genital fungal infections with sodium-glucose cotransporter 2 inhibitor treatment: The ABCD nationwide dapagliflozin audit. Prim Care Diabetes 12(1): 45-50.
- Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, et al. (2019) Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med 380(24): 2295-2306.
- Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, et al. (2020) Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med 383(15): 1436-1446.
- Lo KB, Gul F, Ram P, Kluger AY, Tecson KM, et al. (2020) The Effects of SGLT2 Inhibitors on Cardiovascular and Renal Outcomes in Diabetic Patients: A Systematic Review and Meta-Analysis. Cardiorenal Med 10(1): 1-10.
- Mallik R, Chowdhury TA (2022) Pharmacotherapy to delay the progression of diabetic kidney disease in people with type 2 diabetes: past, present and future. Ther Adv Endocrinol Metab 13: 20420188221081600.
- Rojano Toimil A, Ciudin A (2021) GLP-1 Receptor Agonists in Diabetic Kidney Disease: From Physiology to Clinical Outcomes. J Clin Med 10(17): 3955.
- Nauck MA, Meier JJ (2016) The incretin effect in healthy individuals and those with type 2 diabetes: physiology, pathophysiology and response to therapeutic interventions. Lancet Diabetes Endocrinol 4(6): 525-536.
- Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, et al. (2017) Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 377(13): 1228-1239.
- Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, et al. (2019) Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 394(10193): 121-30.
- Mann JFE, Ørsted DD, Brown-Frandsen K, Marso SP, Poulter NR, et al. (2017) Liraglutide and Renal Outcomes in Type 2 Diabetes. N Engl J Med 377(9): 839-848.
- Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, et al. (2016) Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med 375(19): 1834-44.
- Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, et al. (2019) Cardiovascular, mortality and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol 7(10): 776-785.
- Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ (2017) Cardiovascular Actions and Clinical Outcomes With Glucagon-Like Peptide-1 Receptor Agonists and Dipeptidyl Peptidase-4 Inhibitors. Circulation 136(9): 849-870.
- Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR, et al. (2006) Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 55(6): 1832-1839.
- SPRINT Research Group, Wright JT, Williamson JD, Whelton PK, Snyder JK, et al. (2015) A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med 373(22): 2103-2116.
- ACCORD Study Group, Cushman WC, Evans GW, Byington RP, Goff DC, et al. (2010) N Engl J Med 362(17): 1575-1585.
- (2019) 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2019. Diabetes Care 42(Suppl 1): 103-123.
- Taler SJ, Agarwal R, Bakris GL, Flynn JT, Nilsson PM, et al. (2013) KDOQI US commentary on the 2012 KDIGO clinical practice guideline for management of blood pressure in CKD. Am J Kidney Dis. 62(2): 201-213.
- Lv J, Perkovic V, Foote CV, Craig ME, Craig JC, et al (2012) Antihypertensive agents for preventing diabetic kidney disease. Cochrane Database Syst Rev 12: CD004136.
- Haller H, Ito S, Izzo JL, Januszewicz A, Katayama S, et al. (2011) Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med 364(10): 907-917.
- Bangalore S, Fakheri R, Toklu B, Messerli FH (2016) Diabetes mellitus as a compelling indication for use of renin angiotensin system blockers: systematic review and meta-analysis of randomized trials. BMJ 352: 438.
- de Galan BE, Perkovic V, Ninomiya T, Pillai A, Patel A, et al. (2009) Lowering blood pressure reduces renal events in type 2 diabetes. J Am Soc Nephrol 20(4): 883-892.
- Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, Cleary P, et al. (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329(14): 977-986.
- de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, et al. (2004) Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int 65(6): 2309-2320.
- Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, et al. (2001) Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345(12): 861-869.
- Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, et al. (2001) Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345(12): 851-860.
- ONTARGET Investigators, Yusuf S, Teo KK, Pogue J, Dyal L, et al. (2008) Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 358(15): 1547-1559.
- Frimodt-Møller M, Persson F, Rossing P (2020) Mitigating risk of aldosterone in diabetic kidney disease. Curr Opin Nephrol Hypertens 29(1): 145-151.
- Agarwal R, Kolkhof P, Bakris G, Bauersachs J, Haller H, et al. (2021) Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine 42(2): 152-161.
- Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, et al. (2015) Effect of Finerenone on Albuminuria in Patients With Diabetic Nephropathy: A Randomized Clinical Trial. JAMA. 314(9): 884-894.
- Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, et al. (2020) Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N Engl J Med 383(23): 2219-2229.
- Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, et al. (2021) Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N Engl J Med 385(24): 2252-2263.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.